
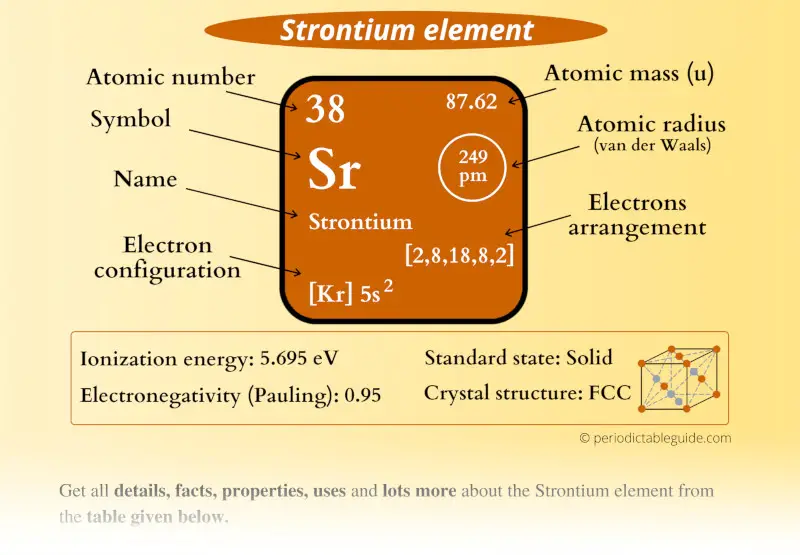

Strontium (Sr), barium (Ba), and radium (Ra). Group 2A (or IIA) of the periodic table are the alkalineĮarth metals: beryllium (Be), magnesium (Mg), calcium (Ca), Closer Look at Neutron Star Crash Demystifies Huge Stellar Explosionsįollow Charles Q.Ancient Neutron-Star Crash Made Enough Gold and Uranium to Fill Oceans.Cosmic Cocoon Spawned by Powerful Neutron Star Crash.
The scientists detailed their findings in the Oct. Still, within the next few years, he and his colleagues hope to collect data that might help them detect other heavy elements within kilonovas, he said. Now, it might be challenging for scientists to detect other heavy elements from neutron star collisions, as there is little quality data on the atomic structures of heavy elements, given their complex nature, Watson said. "This tells us a bit more about what's happening within neutron stars, and what occurs during such mergers." "In order to create a relatively light heavy element like strontium, you need to destroy some neutrons first - you need to bombard them with neutrinos, enough to make them decay more quickly into protons and electrons," Watson said. The key behind this surprising find might be linked with ghostly particles known as neutrinos, which normally pass through conventional matter but can occasionally collide with protons or neutrons. In previous research, scientists expected to find "heavier heavy elements," or heavier r-process elements, "when looking at a kilonova," Watson said. This discovery was surprising because, while strontium is a heavy element, it is also one of the lightest r-process elements. "The fact that we can detect any element in this radioactive explosion is quite surprising," Watson said. Because of its structure, the electrically charged version of strontium produces two powerful spectral lines in blue and infrared light. The key behind this research team's success was strontium's atomic structure, which is relatively simple for such a heavy element. Strontium compounds even help to give fireworks a brilliant red color. On Earth, strontium is found naturally in soil and is concentrated in certain minerals. However, by reanalyzing data from the 2017 merger, Watson and his colleagues have now identified the signature of the heavy element strontium within the fireball. "We could never tell one element from another." This is because "heavier elements can produce blends of tens of millions of spectral lines," Watson said. Prior work suggested the presence of heavy elements within the kilonova, but until now, astronomers could not pinpoint individual elements in the aftermath. They focused on the wavelengths of light, or spectral lines, that, through spectroscopy, scientists have linked to specific elements. Watson and his colleagues suspected that, if heavier elements did form during GW170817, signatures of those elements might be detected in the explosive aftermath of the merger, known as a kilonova. "This explosion was traveling like 30% of the speed of light, so it went from about 100 kilometers in size to the solar system's size in a day," Watson said. Following the discovery of this merger, dubbed GW170817, scientists continued to make telescopic observations from Earth. The scientists made the discovery by detecting gravitational waves, or ripples in the fabric of space-time, which radiated from a collision that happened about 130 million light-years from Earth. In 2017, astronomers witnessed, for the first time, a pair of neutron stars merging. The name neutron star comes from how their gravitational pull is strong enough to crush protons and electrons together to form neutrons. Prior work suggested that a likely source of r-process elements could be the catastrophic aftermath of mergers between neutron stars, which are the superdense cores of stars left behind after cataclysmic, explosive star deaths known as supernovas. Such rapid neutron capture, known as the " r-process" for short, only happens in nature in extreme environments where atoms are bombarded by large numbers of neutrons. Previous research suggested a key clue: For atoms to grow to massive sizes, they needed to quickly absorb neutrons. But how elements heavier than iron, such as gold and uranium, were created has long been uncertain.


 0 kommentar(er)
0 kommentar(er)
